Abstract
Background: The introduction of novel therapeutics has led to improved outcomes in patients with multiple myeloma (MM). MM and its precursor lesion smoldering multiple myeloma (SMM) have traditionally been associated with increased mortality despite treatment. We aimed to assess the impact of a diagnosis of SMM and MM compared to the general population in the context of established prognostic factors.
Methods: We studied the overall survival of 1697 patients with smoldering multiple myeloma (SMM, n = 582) and multiple myeloma (MM, n = 1115) diagnosed at Mayo Clinic between 01/2005 and 12/2015. Expected survival accounting for age and sex was calculated using the United States general population (US) as the reference group. Observed and expected overall survival was expressed as the standardized mortality ratio (SMR) of observed to expected deaths. Kaplan-Meier overall survival estimates were calculated and the log-rank test was used to compare overall survival in subgroups. The subgroups of interest were based on the International Staging System (ISS) and the presence of fluorescence in situ hybridization (FISH) high-risk cytogenetics: t(4;14), t(14;16), t(14;20), and del(17p). Proportional hazards regression models were used to assess the associations between the aforementioned prognostic factors and overall survival. P-values below 0.05 were considered statistically significant.
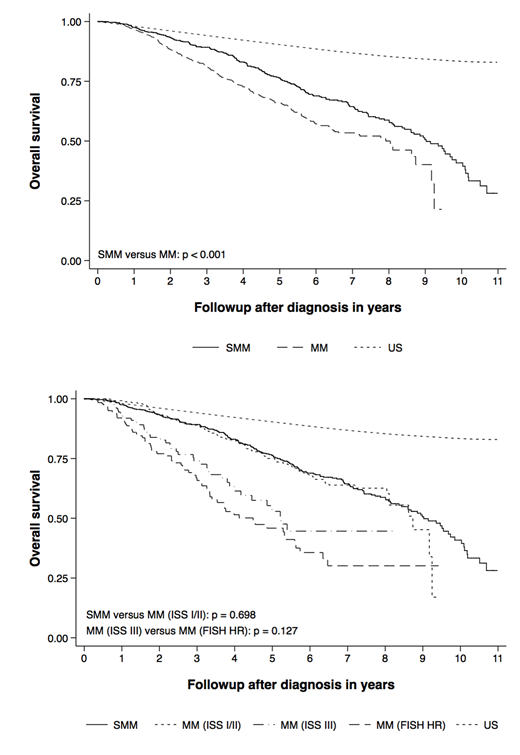
Results: The median age at diagnosis in patients with SMM and MM was 65 (32 - 92) and 63 years (24 - 90), respectively. Two hundred forty-nine patients (57%) and 663 (60%) were male. The median follow-up in patients with SMM and MM was 4.7 (0.1 - 11.0) and 2.6 years (0.2 - 9.5). The median overall survival for patients with SMM and MM was 9.0 (95% CI 8.4 - 9.7) and 7.9 years (6.4 - 8.7). With age- and sex-matched population controls as the reference, the SMRs in patients with SMM and MM were 2.6 (95% CI 2.2 - 3.0) and 4.6 (4.1 - 5.2). Among those MM patients with available data on ISS staging and FISH cytogenetics, 30% (236/780) had ISS III and 21% (188/878) had high-risk cytogenetics. Patients with MM (compared to SMM) experienced worse overall survival (HR 1.5, 95% CI 1.2 - 1.8, p < 0.001, n = 1697). Patients with ISS I/II MM (compared to SMM) experienced similar survival (HR 1.0, 95% CI 0.8 - 1.3, p = 0.698, n = 1131). Patients with ISS III MM (compared to MM with high-risk cytogenetics) experienced similar survival (HR 1.3, 95% CI 0.9 - 2.0, p = 0.128, n = 329).
Conclusions: Patients with SMM and MM in this cohort experienced excess mortality compared to the general population. In the absence of universal screening the true morbidity and mortality of patients with SMM and MM remains unknown and is likely overestimated in hospital-based cohorts. Overall survival in patients diagnosed with SMM and patients with ISS I/II MM receiving contemporary anti-myeloma therapy was clinically indistinguishable. Patients treated for ISS III MM experienced overall survival similar to patients with cytogenetic high-risk disease. MM remains associated with excess mortality, the magnitude of which varies considerably based on the presence of additional tumor and host factors. The outcomes with modern therapy among the ISS I/II patients highlight the potential for improving outcomes of SMM by early intervention, especially for the higher risk patients.
Lacy:Celgene: Research Funding. Gertz:Medscape: Consultancy; Alnylam: Honoraria; janssen: Consultancy; spectrum: Consultancy, Honoraria; Ionis: Honoraria; annexon: Consultancy; celgene: Consultancy; Teva: Consultancy; Prothena: Honoraria; Apellis: Consultancy; Abbvie: Consultancy; Physicians Education Resource: Consultancy; Amgen: Consultancy; Research to Practice: Consultancy. Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Russell:Vyriad: Equity Ownership. Kapoor:Takeda: Research Funding; Celgene: Research Funding. Kumar:Roche: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal